I’d like to start with a brief description of wine acids and pH. First, looking at first figure, you can see four of the acids commonly found in wines. There are the two major acids, tartaric acid, designated with a “T”, and malic acid, designated with an “M”. Both are dicarboxylic acids, meaning they each have two hydrogen ions that can react easily. Next we have lactic acid, designated by an “L”. It is a monocarboxylic acid. The fourth acid shown is citric acid, designated by a “C”. This acid is a tricarboxylic acid.
I’d like to start with a brief description of wine acids and pH. First, looking at first figure, you can see four of the acids commonly found in wines. There are the two major acids, tartaric acid, designated with a “T”, and malic acid, designated with an “M”. Both are dicarboxylic acids, meaning they each have two hydrogen ions that can react easily. Next we have lactic acid, designated by an “L”. It is a monocarboxylic acid. The fourth acid shown is citric acid, designated by a “C”. This acid is a tricarboxylic acid.
pH
I’d like to also clarify some definitions. Looking again at figure 2, if we were to measure all the hydrogen ions floating freely, those shown with blue shading, we would be measuring the pH. Thus, pH is a measure of the free H+ ions. The numerical pH value is the logarithm of the concentration of the free hydrogen ions in a solution. A lower pH number is equivalent to a higher acid strength. A solution with a pH of 3.0 has 10 times the free H+ ions as a solution with a pH of 4.0.
Titratable Acidity
If next we were to perform a titration on our sample with a solution of sodium hydroxide, the sodium hydroxide would react with all the hydrogen ions shown, both those in the blue shaded circles and those in the open circles. This measure is called the titratable acidity. The total acidity of a wine, must or juice sample is an entirely different measure. In figure 2, total acidity is the sum of the concentration of all of the acid cations, or all of the T’s, M’s, and C’s, and if they are present, L’s. It is not a measure generally used in winemaking.
In the United States titratable acidity is usually reported as g of tartaric acid/L, measured in a titration to an endpoint of pH 8.2. Some countries titrate to an endpoint of pH 8.0, or pH 8.3. All three endpoints yield similar values, generally within 5% of each other.
Sometimes the titratable acidity is reported as a percent, which is really g of tartaric acid/100 mL. To convert from percent to g/L, multiply the percent value by 10.
0.7 % TA = 7.0 g/L TA
Some scientists and some winemakers report TA using the term meq/L. If you see these types of values, divide the value in meq/L by 13.33 to get TA in g/L.
100 meq/L TA = 7.5 g/L TA
In some European countries the convention is to report TA as g of sulfuric acid/L. This gives a numerical value which is 2/3 of the numerical value we would report. To convert g of sulfuric acid/L to g of tartaric acid/L, multiply by 1.5.
5.0 g sulfuric acid/L TA = 7.5 g tartaric acid/L
Why are pH and TA (titratable acidity) important in winemaking?
The pH of wine is the major variable in the taste of sourness. With everything else equal, the lower the pH of a wine, the more sour it tastes. The pH and the TA both influence the sensation of astringency. Along with tannins they balance the sweetness of wine that results from the sugars, and the alcohol and glycerol concentrations, and help make wine a pleasant beverage to drink. Balanced pH and acidity also enhance the fruit character of a wine.
The pH has major influences beyond taste. At higher pH levels, the appearance of a wine is adversely affected. Red wine color intensity decreases, and more brown hues appear. This browning is especially unattractive in white wines. Additionally, there is a restricted capacity for a wine to mature.
High pH wines also leave a number of the chemical components in an ionized state. This leaves them much more susceptible to oxidation. A high pH red wine is likely to lose quality after just a few years.
High pH wines are also more troublesome to process. Undesirable lactic acid bacteria and acetic acid bacteria (think volatile acidity!) show much greater vigor at high pH than at pH’s on the range of 3.1 – 3.6. Also with a high pH, it is harder to prevent unwanted lactic acid bacteria (LAB) from growing during the early stages of primary fermentation, and it is harder to control them during desired malolactic fermentation. Remember, too, that the antibacterial effects of sulfur dioxide and of fumaric acid are reduced rapidly as the pH increases.
A wine’s clarity is also affected by pH. High pH wines are more susceptible to protein instability. The effectiveness of bentonite fining is reduced as the pH is increased.
What are the major acids of winemaking?
Tartaric acid is the acid usually found at the highest concentration in wine. Its level runs from 6+ g/L in grape musts from cooler climates, to 2 – 3 g/L in musts from warm climates. Its concentration in grapes is relatively unchanged during maturation. Its concentration is also unaffected by the metabolic processes of primary fermentation or secondary fermentation, but its solubility is affected by the concentration of alcohol.
L-Malic acid is the second major acid of grape wines. It’s concentration can run from 4 – 6.5 g/L in grapes from cool climates, to 1 – 2 g/L in musts from warmer climates. Its level decreases during maturation due to berry respiration. While normally not metabolized by primary fermentation yeasts, some yeast strains can reduce L-malic acid concentrations by 20 – 40% (known as maloalcoholoic fermentation). During secondary, or malolactic fermentation, lactic acid bacteria convert essentially 100% of remaining natural L-malic acid to L-lactic acid, reducing the titratable acidity by 0.5 – 3.0 g/L.
Citric acid is a minor acid of grapes, appearing at levels up to 10% of the total acid content, or 0.1 – 0.7 g/L. Citric acid is produced by yeasts during primary fermentation (at ~0.1 – 0.4 g/L), dependent upon yeast strain used. Citric acid is broken down by lactic acid bacteria during malolactic fermentation, producing diacetyl, a flavor component. Some diacetyl (e.g., up to 4 mg/L) is considered good, but too much diacetyl is considered a flavor defect. Citric acid can also be broken down by unwanted LAB, producing excess diacetyl and increasing acetic acid (volatile acidity).
Lactic acid is not found naturally in grape musts. It can exist in two forms, L-Lactic acid and D-lactic acid. Both forms are produced at low levels (0.05 – 0.20 g/L) by yeast during primary fermentation. During malolactic fermentation the L-lactic acid form is produced when lactic acid bacteria metabolize malic acid. Levels produced are equivalent to 50% of the malic acid present at the start of malolactic fermentation, and can reach upwards of 3 g/L. D-lactic acid can also reach levels of 0.3 g/L and higher as a result of fermentation of residual sugars by contaminating forms of lactic acid bacteria.
Succinic acid is not found naturally in grape musts either. It is present in most wines. About 1 g/L is produced during primary fermentation. It is undesirable at high levels because of its bitter, salty taste.
What are the optimum pH and Acidity?
For table wines, preferred pH levels are 3.1 – 3.4 for white wines, and 3.3 – 3.6 for red wines. Preferred titratable acidity levels are 7 – 9 g/L for white wines, and 6 – 8 g/L for red wines.
When a juice or must is outside of the optimum ranges above, an adjustment should be considered. An adjustment which lowers the pH should be considered for pH values above 3.7, especially as a way to discourage microbial spoilage. Remember that titratable acidity is likely to increase about 10% during primary fermentation with standard yeasts as a result of the formation of succinic acid.
How can I increase titratable acidity levels?
- Tartaric acid is the principal acid used when considering an acid addition. A tartaric acid addition usually lowers the pH, and is significantly more effective than malic acid at lowering pH. Tartaric acid additions will have their greatest impact on acid levels when they are made before primary fermentation. Remember that potassium bitartrate has only moderate solubility in alcoholic solutions, so some of the acid added is likely to precipitate. If the pH is below 3.7 prior to acid addition, a tartaric acid addition will likely result in a beneficial reduction in pH. This is partly due to the removal of some of the potassium present in the must. If the grapes/juice are from warmer climates and have a high pH and low acidity, a mixed treatment may be better than tartaric acid addition alone.
- Malic acid can also be added as an adjustment prior to primary fermentation. It is less likely to cause a shift in pH, but it is also less likely than a tartaric acid addition to result in precipitation of potassium bitartrate. Note that malic acid comes in two forms, D-malic acid and L-lactic acid.
DL-malic acid, a mixture of D-malic acid and L-malic acid, is the form most readily available commercially, and is the most economical. In DL-malic acid, the D-malic acid form will affect the acidity, but it will not later undergo malolactic fermentation. Thus, only about half can later be converted; the other half is microbially stable. It should be added that the D-malic acid form has caused a slowing of primary fermentation by some strains of yeast. Also, especially when MLF is not planned, with malic acid additions there is a risk of developing green acid taste. (Remember Granny Smith apples.)
The L-malic acid form is more expensive, and will cost about $0.50/bottle of wine for a 1 g/L addition. It, too, will not cause potassium bitartrate precipitation. The added L-malic acid is all available for malolactic fermentation. This type of addition may be warranted for a wine with lower than desired malic acid levels, but one that is known to benefit from the “softening” provided by malolactic fermentation.
Malic acid and tartaric acid are the only acids that can be added prior to primary fermentation. They can be used as acidulants post-fermentation as well.
- L-Lactic acid can be added directly to improve mouth feel, as can be achieved with malolactic fermentation. It, too, is unlikely to cause bitartrate precipitation. An L-lactic acid addition should be made after primary fermentation.
- Citric acid has occasionally been used as an acidulant. It is generally not recommended. It can be broken down by lactic acid bacteria, producing excess diacetyl and troublesome amounts of acetic acid. It is useful at levels of 100 – 120 mg/L to react with excess iron in wine and reduce the likelihood of iron casse developing. Otherwise it is best restricted to use with white wines not undergoing malolactic fermentation, with the addition being made shortly before filtration and bottling.
- Fumaric acid can be used as an acidulant. Were it added before primary fermentation it would be metabolized. Fumaric acid is inhibitory to lactic acid bacteria at the 1.5 – 2.0 g/L level, and can be used in conjunction with sulfite. As with sulfite, its effectiveness decreases with increasing pH.
- Sorbic acid is usually used as a fungistat rather than as an acidulant or bacteriostat. It is authorized by the EC at only 0.2 g/L, which is at the low end of its effective range. It can produce unpleasant “geranium” odors when acted on by lactic acid bacteria during malolactic fermentation or afterwards. It has found use in sweet wines to prevent refermentation, and is used in conjunction with a bacteriocide or bacteriostat like sulfur dioxide.
How can I decrease acidity levels?
When grapes are grown in cooler climates they frequently have high titratable acidity, and malic acid levels are as high as tartaric acid levels. Here are some approaches to reducing acid levels.
- Carry out primary fermentation with a malic acid-metabolizing yeast strain, conducting the process of maloalcoholic fermentation. Some strains of Saccharomyces cerevisiae (e.g., Lalvin 71B or Lalvin AC) can metabolize 20 – 40% of the malic acid present in a must, producing alcohol instead of lactic acid. These strains reduce the amount of malic acid available for MLF, and lowering the risk of getting too much “mouth feel improvement.” These strains offer reduced risk of losing varietal characters. Some strains of Schizosaccharomyces pombe (e. g., Lallemand ProMalic) will reduce 100% of the malic acid in a must during primary fermentation. With these strains there is some risk of producing off flavors, so yeast removal is performed immediately following completion of primary fermentation. Maloalcoholic degradation of malic acid does not result in a change in pH.
- Perform chemical deacidification with calcium carbonate. In chemical deacidification with calcium carbonate, the calcium reacts directly with tartaric acid, producing insoluble calcium tartrate. The carbonate is converted to CO2, and released to the atmosphere. In this process only the tartaric acid component of acidity is affected. Adding about 0.7 g/L of calcium carbonate will reduce the titratable acidity by about 1.0 g/L, but note that it is also likely to increase the pH by about 0.1 pH unit. Initial calcium tartrate precipitation is rapid, but there is some risk of calcium tartrate precipitate occurring after bottling, causing a visual defect. Another risk with this procedure is the formation of calcium malate, which is soluble. Calcium malate can impart an unpleasant salty taste to wine. Calcium carbonate deacidification is best applied with when desired acidity changes are large, and before or during primary fermentation. Perform laboratory trials first.
- Perform deacidification with potassium bicarbonate. Chemical deacidification with potassium bicarbonate works similarly, producing insoluble potassium bitartrate and releasing CO2 to the atmosphere. It, too, works only on the tartaric acid component, and is likely to cause an increase in pH. Potassium bitartrate precipitation is slower than calcium tartrate precipitation, but with cold stabilization there is less risk of any precipitate forming after bottling. This technique is best applied when only small changes in acidity are desired.
- Treat wine with Acidex. Acidex treatment is a method for reducing both the malic acid and the tartaric acid components in roughly equal amounts. This procedure does require some additional manipulation. A portion of the wine is separated, and calcium carbonate is added to raise the pH to 4.5 – 4.8. (Be careful not to raise the pH of this portion too much higher or it will become susceptible to significant undesirable oxidation.) Acidex is next added to the treated portion, causing the precipitation of the calcium malate tartrate double salt. The treated portion is filtered to remove the precipitate, then the filtered portion is reblended with the untreated portion. Acidex treatment works best in white wines when performed after clarification but before primary fermentation. For red wines, apply the Acidex treatment at the end of alcoholic fermentation.
- Conduct malolactic fermentation. MLF is a method for reducing the acidity due to malic acid by about 50%. This is accomplished by lactic acid bacteria converting L-malic acid to L-lactic acid. This process produces some additional flavor components, and also releases enzymes which, in turn, will later release aroma components that are chemically bound to sugars. The MLF process is recommended for most red wines and some white wines, especially Chardonnay. It is important to monitor the completion of this process, however, since following conversion of the malic acid, the lactic acid bacteria can produce acetic acid (VA) from remaining hexose and pentose sugars, and can produce excess diacetyl from citric acid. At completion of the MLF process, sulfite can be added to control the lactic acid bacteria. The pH will usually increase 0.1 – 0.2 pH units as a result of malolactic fermentation.
- Blend the high acid wine with a low acid wine. This method works well with regard to expected acidity levels. Unfortunately, low acid wines are not always available. Also, stability of the resulting wine may be adversely affected.
- Perform stabilization. This process involves chilling a wine and holding it at low temperatures (just above the freezing point of wine). This causes precipitation of potassium bitartrate crystals. The process will reduce only the tartaric acid component, and will also reduce potassium levels as well. The pH of the resultant stabilized wine will decrease 0.1 – 0.2 pH units if the starting pH is below 3.65. If the starting wine is above a pH of 3.65, the resulting wine’s pH will increase 0.1 – 0.2 pH units. (This step is a normal winemaking operation.)
- Perform amelioration. Adding water will reduce the acidity of a must or wine. It is generally not allowed in commercial winemaking. It will dilute flavor components, aromas, and color intensity as well as the acidity.
How do I adjust pH?
In most cases, pH will change, or not change, as a direct result of the chemical reactions that occur during the acidification and deacidification procedures discussed above.
There is one process, called ion exchange, which can be used to adjust the pH of a wine without affecting the titratable acidity. The procedure involves passing wine through a column containing a cation exchange resin. The resin replaces potassium ions in the wine with hydrogen ions, effectively reducing the pH. Although it has been around for over fifty years, ion exchange is not yet widely used in winemaking. Most recently it has
been applied to pH reduction in Cynthiana wines.
A second method for direct pH adjustment is addition of food grade “mineral acids”, such as sulfuric acid or phosphoric acid. These acids lower pH more and acidity less than equivalent amounts of tartaric acid. It should be noted that these chemicals may not be allowed under current commercial winemaking regulations.
What about those acids I don’t want?
Above we discussed the major acids found in grapes and grape wines. Tartaric acid and malic acid, with citric acid and L-lactic acid are generally desirable when their concentrations are within broad limits. We also learned how to adjust their levels. Below is some information on acids we want to control, i.e., limit, in wine
D-Lactic Acid
Lactic acid bacteria (LAB) are important in wine production. They are responsible for the malolactic fermentation that occurs in many wines, reducing the wine’s total titratable acidity, softening the wine, producing a broader palate of flavors, and enhancing the wine’s microbiological stability. The lactic acid bacteria found in wine fall into four genera: Lactobacillus, Leuconostoc, Pediococcus, and Oenococcus, which is most frequently responsible for malolactic fermentation. Conventionally, they are further identified by their metabolic preferences:
| General Class | Genus | Basic Carbohydrate | Major Metabolic Product(s) |
| heterofermenters | Lactobacillus | glucose | lactic acid + acetic acid + ethanol + carbon dioxide |
| Leuconostoc | |||
| Oenococcus | |||
| homofermenters | Pediococcus | glucose | lactic acid (> 85%) |
| facultative heterofermenters | P. pentosaceus | glucose (or) pentoses (e.g., arabinose) | lactic acid |
| L. plantarum, etc | lactic acid + acetic acid |
Of these wine LAB, all Leuconostoc sp., all Lactobacillus sp. except L. casei (usually found in milk products), and all Pediococcus sp. at wine pH levels produce D-lactic acid from sugars.
Unfortunately, these same bacteria may inhibit primary alcoholic fermentation, and can also cause development of a range of off flavors known as lactic taint, piqûre lactique in France, and spunto lattico in Italy.
How does this occur? It’s related to bacterial metabolic pathways. Under preferred conditions of moderate wine pH, sufficient nutrients, warmer temperatures, and low carbohydrates (sugars), lactic acid bacteria (LAB) convert L-malic acid to L-lactic acid, and deliver the improvements listed above. However, under many other conditions, such as stuck or sluggish fermentation, the malolactic LAB and/or contaminating LAB convert
some of the remaining sugars into D-lactic acid and acetic acid, one of the major “incidents” in winemaking. They can generate acrolein from glycerol which in turn reacts with anthocyanins to give wine a bitter taste. They cause “ropy” wines by converting residual sugars into long chain polymers that result in an abnormal and unacceptable increase in viscosity. They produce excess levels of buttery diacetyl, cause “mousy” wines, and produce ethyl carbamate from arginine. They can form biogenic amines like histamine, tyramine, and putrescine, chemicals which cause allergic reactions in susceptible individuals. Under the right (wrong?) conditions LAB can metabolize the preservative sorbic acid, and give wine the aroma of geraniums.
Where do LAB come from? Many strains of LAB have been found at low levels on grapes. Where fruit damage has occurred, levels are considerably higher. In one study, LAB were found on 9 of 21 batches of undamaged grapes, but on 16 of 22 batches with damaged grapes. In another study, when sampled during fermentation, 31% of Oregon wines were found to contain detectable levels of Lactobacillus sp., with one winery showing a contamination rate of almost 80%. LAB can also be introduced from inadequately sanitized pumps, valves, and transfer lines, as well as from almost-impossible-to-sterilize cooperage. Given the conditions at crush of high sugars, warmer temperatures, and higher than desired pH levels, LAB can multiply rapidly. They generally decrease during primary fermentation as a result of their susceptibility to higher alcohol levels, but LAB populations can reemerge at troublesome levels during malolactic fermentation, or during aging and storage, if protections are inadequate. Monitor often! Grapes themselves do not produce lactic acid. D-lactic acid, at levels above 300 mg/L, is related to contamination!
How do we control them? Most LAB generally do not grow in lower pH wines; they exhibit reduced growth in wines with a pH below 3.5, and essentially no growth when the pH is below 3.2, a corrective action available with white wines but typically not reds. Higher alcohol levels (>13%) slow their growth, but are not lethal. Many LAB are susceptible to SO2, especially at total SO2 levels above 70 ppm; however, since the Oenococcus oeni sp. usually preferred for malolactic fermentation are more susceptible to SO2 than Pediococcus sp. and Lactobacilli, alternate methods of control should be considered if MLF is planned. Lysozyme has been found useful for control of LAB at levels of 100 – 250 mg/L, especially during crush, cold soak, and primary alcoholic fermentation when malolactic fermentation is planned. Contaminating LAB are also fairly susceptible to temperature. Control the temperature during malolactic fermentation at 20 – 23•C, and at 15 – 18•C afterwards.
Since grapes do not produce lactic acid, regular monitoring throughout winemaking and aging, i.e., regular measurement of D-lactic acid levels, can be used as an indicator of the onset of bacterial contamination and allows for minimally invasive yet effective control of lactic acid bacteria infections. The uncontrolled bacterial activity of lactic acid bacteria in wines can cause a drastic alteration of the quality of the final product!
Acetic acid
Acetic acid is the main volatile acid in wine. It is formed during yeast fermentation as a side reaction of acetaldehyde oxidation. The range of formation is 200 – 400 mg/L. At this level it is not noticeable on the palate and has no effect on wine quality. Acetic acid can be produced by lactic acid bacteria, as mentioned above. It is also formed by acetobacter spoilage in an aerobic condition. The bacteria Acetobacter and Gluconobacter use ethanol (and glucose) aerobically in the formation of acetic acid. Thus growth of acetic acid bacteria in wine as well as in musts and on deteriorating grapes may significantly increase the volatile acidity content. Above 600 – 700 mg/L acetic acid/volatile acidity is noticeable and it depreciates wine quality.
The legal limits in the U.S. for volatile acidity are 1,200 mg/L for white wines and 1,400 mg/L for red wines. For California, the limits are 1,200 mg/L for red wines and 1,100 mg/L for white and dessert wines, while the OIV has a limit of 980 mg/L for red and white wines.
Unlike lactic acid bacteria and yeasts, acetic acid bacteria generally serve no purpose useful to the winemaker. They have as a key metabolic feature the ability to form acetic acid and ethyl acetate from ethanol. They are members of the bacteria family Acetobacteraceae, and fall into the following genera:
Regarding their metabolism, all acetic acid bacteria require oxygen, i.e., they are aerobic. However, many are able to survive, and even grow, without any oxygen present. Also, here’s the key metabolic difference between the two genera found in wine: Acetobacter can further convert acetic acid to CO2 and water, while Gluconobacter and Gluconoacetobacter cannot.
Where do acetic acid bacteria come from? Many species of acetic acid bacteria are present on grapes, and accompany them into the winery at harvest. They continue to grow in musts that have not been sulfited, but are generally greatly reduced during fermentation as a result of the anaerobic conditions. (Note that in some studies new strains of AAB, not found on the grapes, have been identified at the end of primary fermentation, most likely introduced via contamination from within the winery.) Following fermentation in the absence of efforts for control, acetic acid bacteria show renewed growth as a result of exposure to oxygen.
How can we control them? Acetic spoilage is primarily related to storage conditions! Since acetic acid bacteria require a great deal of oxygen to convert ethanol to acetic acid, maintaining wine in sealed, toppedoff containers goes a long way in controlling them. As with LAB, AAB are much less active at low pH. Spoilage is almost non-existent at pH 3.0, but can occur at pH 3.4 and above. AAB are sensitive to temperature, and do not grow well at preferred storage temperatures around 15ºC. The best control of acetic acid bacteria is sulfite.
Sulfurous Acid, a.k.a. SO2
Sulfur dioxide is a chemical important in the production of wine. It is widely and effectively used as a preservative, and this is its primary function. Why then is it so difficult to understand? Let’s see if we can conquer sulfur dioxide by breaking it down into several factors.
First, where does sulfur dioxide come from? There are two primary sources for sulfur dioxide, or SO2, in wine. One source is from yeast. Different strains of yeast can produce anywhere from 10 ppm to 80 ppm during primary alcoholic fermentation. Most commercial strains for winemaking produce only 10 – 30 ppm, so they are generally preferred. The amount of SO2 produced by a given strain of yeast is also influenced by certain chemicals such as methionine and cysteine, but since these nutrients come from the grape juice itself, we need not be concerned about them. The second source of SO2 is from additions made by the winemaker.
Second, what does SO2 do? At the levels usually desired in wine, sulfur dioxide does several things. When added early to grapes or juice, especially with white wines, it inhibits some of the enzymes that cause browning. This can be especially helpful if a pre-inoculation “cold soak” is planned for a delicate white wine such as a Muscat. Another reason for addition of SO2 before primary fermentation is to control “wild” yeasts that are present on the grapes, but are not species usually found helpful in fermentation to wine alcohol levels. Sulfur dioxide added before primary fermentation, after primary fermentation, or after malolactic fermentation can be used to control the growth of bacteria. But note, if malolactic fermentation is desired,
addition of sulfur dioxide before malolactic inoculation must be made at relatively low levels since malolactic bacteria are inhibited at moderate levels of Free SO2.
Third, where does sulfur dioxide go? Not all of the SO2 that starts off in the wine stays in the wine. Some, but not much, is given off as a gas. Some gets bound to other chemicals in wine, such as acetaldehyde and anthocyanins and even residual sugars. Some chemically reacts with oxygen and other “oxidizing substances.” And some, the “Free SO2,” stays around to work. There are a couple of other points to make here.
Most of the bound sulfur dioxide, which can be 50 – 90% of the amount of SO2 added, is not available to function as a preservative. It is part of the “Total SO2” in a wine, but is effectively of no use as a protectant against oxidation or against bacteria. That is why most measurements are for “Free SO2.” Also, since sulfur dioxide protects against oxidation, especially of red wines, why not just add a bunch as long as you can’t taste it? The problem here is that too much SO2 ties up the aldehydes in wine, a chemical family that slowly reacts with tannins over time to produce the desired improvements in a wine’s taste. You wouldn’t have oxidized wines, but you would have overly astringent wines instead. That’s part of the reason for the upper end on the target levels for Free SO2.
Fourth, what’s the big deal with pH? Well, let’s start with a little chemistry. The sulfur dioxide in wines exists in two different chemical forms, SO2 and HSO3 -1. These two chemical species are called “molecular SO2” and “bisulfite,” respectively. When you measure Free SO2 you measure both the molecular SO2 and the bisulfite, but only the molecular SO2 is effective as a bacterial preservative! And in wines, only 1% to 7% of the Free SO2 is present as molecular SO2. These two chemical forms are related in the following chemical equation:
SO2 + H2O = HSO3-1+ H+1
What this equation says is that if you have more acid, which is the same as having a low pH wine, then you get a higher percentage of the molecular SO2. If you have less acid, or a high pH wine, you get a much lower percentage of the molecular SO2. The following table shows the percent of the Free SO2 that is present as molecular SO2 for different pH levels in wine.
| pH of wine | % as molecular SO2 |
| 3.0 | 6.06 |
| 3.1 | 4.88 |
| 3.2 | 3.91 |
| 3.3 | 3.13 |
| 3.4 | 2.51 |
| 3.5 | 2.00 |
| 3.6 | 1.60 |
| 3.7 | 1.27 |
| 3.8 | 1.01 |
| 3.9 | 0.81 |
| 4.0 | 0.64 |
What the table says is that you need 9.45 times as much Free SO2 if your wine has a pH of 4.0 as you would if the same wine had a pH of 3.0. Wow! Do I have to understand all this chemistry to make wine? Not exactly, but it’s helpful to understand the concepts given above, then use the included chart entitled “SO2 goals in yellow” to actually calculate your additions.
Now I know how much is needed, but how do I make my additions? First, measure the pH. If the pH is not within the range you want and you are planning a pH adjustment, make the pH adjustment before you add your SO2. Now consult the chart for the pH of your wine, then pick the target SO2 level you want. For example, if your red wine has a pH of 3.4, you’ll choose a target of 32 ppm, and use this figure in your addition calculation. However, if you added some sulfur dioxide previously you’ll need to perform a Free SO2 test to see what level is present in the wine. If the analysis says you have 10 ppm already present in the wine, then you subtract this from the target amount, and get 32 ppm – 10 ppm = 22 ppm as the amount to add.
O.K., so just how much is 22 ppm. If you will be using potassium metabisulfite for your addition, you can use the following formula:
Grams of metabisulfite to add = ______ppm x 0.00657 x _______gallons of wine, juice or must
Thus, for a 22 ppm addition to 5 gallons of wine, you will add: 22 x 0.00657 x 5 = 0.72 g of metabisulfite.
Remember that SO2 tends not to mix, but stays where it is added in juice or remains in a layer near the bottom in wine. Always stir thoroughly, then stir again the next day. Also, because some of the added sulfur dioxide will become bound and unavailable, wait 2-3 days before retesting so that free SO2 and bound SO2 have a chance to equilibrate, then retest and make another addition if needed. For these reasons it is recommended to make adjustments at least a few days before bottling. (Some winemakers making smaller batches may find it convenient to use Campden tablets instead of potassium metabisulfite. The calculation to use is the same. Just remember that each tablet contains 0.44 g of potassium metabisulfite. Therefore, in
the example above, you would use two Campden tablets.)
How can I Best Control pH, Acids and Acidity?
Monitor! Monitoring is something we are all used to in our daily lives. We get periodic medical checkups to catch problems when they are little and correctable. We get periodic dental checkups to make sure small cavities don’t develop into major ones. We go over our children’s report cards to make sure they are making satisfactory progress and, if not, work with their teachers to bring them up to standard. Monitoring the winemaking process is similarly a way of avoiding surprises and taking corrective action when problems are small and fixable. Some might ask, though, if the trouble of monitoring is worth the effort. To answer this question, let’s look at a cost analysis prepared a few years ago on a wine that was caught unnecessarily in a stuck fermentation. In this analysis, Chardonnay grapes were purchased at $1,500/ton. They yielded 4,000 gallons, a modest size fermentation lot with an investment cost of about $50,000. This wine had a potential return, at $120/case wholesale, of $181,000; however, because of the stuck fermentation and the resultant off-specification wine produced, the actual yield for the wine on the bulk market was $15/gallon, or $51,000. The loss of potential earnings was $130,000 for this one lot alone, a loss that could likely have been avoided with some pre-fermentation testing or some monitoring during the fermentation process. The testing cost would have been under $100.
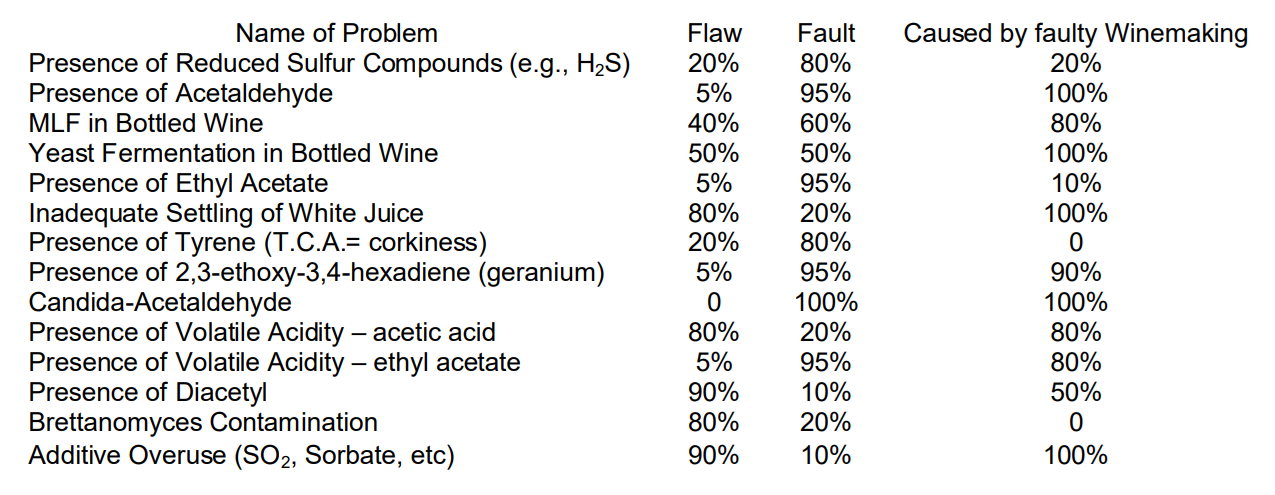
Not all fermentation lots are as large, but the risks of an unfavorable outcome are the same. To reliably manage the winemaking process in order to prevent surprises and make adjustments as needed to produce desired wines, both commercial and home winemakers should employ a basic monitoring scheme. An example of the types of problems that can occur in winemaking was given in a recent article by wine judges evaluating wines made by home winemakers. The problems were listed as either flaws – a defect that is a minor departure from an acceptable norm and one that causes the wine to be atypical and less than normally enjoyable – or a fault – a character experienced as a major departure from an acceptable norm and one that spoils the wine and causes it to be significantly atypical, usually unpleasant, and often undrinkable. These defects were also categorized as to whether they were caused by poor winemaking practices or not.
Clearly, making wine without knowing the status of the winemaking process can result in production of an unpleasant or undrinkable concoction. We can greatly avoid this undesired outcome by following a plan of monitoring.
Harvest Monitoring – Monitoring in winemaking begins in the vineyard. Starting about 6 weeks after veraison, grape sugars should begin to be checked weekly. When the sugar levels (as °Brix) reach 18, monitoring should be expanded to include pH, titratable acidity, and color/tannins, and the frequency of testing should be increased. As grapes reach physiological maturity, sugars continue to rise, pH starts to rise and can reach levels above the preferred pH 3.4 for white wines and pH 3.6 for red wines, and acidity drops as malic acid metabolism/respiration increases. (In grapes approaching an overripe stage, sugars and acids can both increase as a result of grape dehydration.) Color/tannins continue to rise as well, but harvest decisions regarding this parameter are typically made based on prior history as opposed to achievement of a fixed value.
Wine Monitoring – Monitoring in the winery begins with the receipt of grapes, and continues until bottling. Individual monitoring schemes can be developed according to each winemaker’s needs and preferences. One sample of a monitoring plan is given below. Some of the key processes in winemaking and the risks associated with those processes are:
- Upon receipt and crushing, measurement of total fermentable sugars will provide an estimate of final alcohol level. Measurement of acidity and adjustment if needed will ensure best extraction of flavors. Measurement and adjustment of pH will ensure best conditions for management of primary fermentation. Measurement of free SO2 levels, if SO2 is added, will indicate if adequate amounts are present to control unwanted lactic acid bacteria. Measurement and addition of yeast assimilable nitrogen will reduce the risk of stuck fermentation while minimizing the likelihood of ethyl carbamate formation.
- During primary fermentation, monitoring of fermentable sugars, pH, acidity, and temperature on a daily basis will confirm adequate fermentation progress, highlight a situation where a fermentation is running too fast, and flag the onset of a sticking fermentation. (A stuck fermentation can result in the production of undesirable hydrogen sulfide, or leave a wine with exceeding high amounts of unfermented sugar.) Also, sugar measurement will determine the proper time for inoculation with malolactic cultures, if desired, or for addition of controlling levels of sulfite. Acid measurement will indicate the need for any adjustments needed for proper wine balance.
- If malolactic fermentation is desired, measuring L-lactic acid levels will confirm the onset of malolactic fermentation, while monitoring the reduction of malic acid levels will confirm the progress of this fermentation. Also, measurement of malic acid levels at the end of malolactic fermentation will indicate the proper time for addition of controlling levels of sulfite so that the wine in not left unnecessarily under protected. Concurrent monitoring for D-lactic acid will identify the growth of any undesirable lactic acid bacteria, or a malolactic fermentation that has begun to go awry.
- During storage and racking, measurements of pH, titratable acidity, volatile acidity, D-lactic acid, and free SO2 will confirm the wine is behaving in a desired and conventional fashion, or highlight the onset of an undesired bacterial contamination. Remember that when volatile acidity is noticeable by taste or smell, the wine will not even be salvageable as bulk wine in the example given above. Without expensive reverse osmosis reprocessing, it will not have any salvage value. Remember also that Free SO2 is not only eliminated over time by oxidation, but that 40 to 90 % of added SO2 can become bound to other wine components and rendered inactive. This is why wine should be monitored every three weeks during aging.
Remember, the key to making the wine you want the way you want is “to monitor how each production activity affects wine palatability and to make adjustments accordingly.”