Sulfur dioxide is a chemical important in the production of wine. It is widely and effectively used as a preservative, and this is its primary function. Why then is it so difficult to understand? Let’s see if we can conquer sulfur dioxide by breaking it down into several factors.
First, where does sulfur dioxide come from? There are two primary sources for sulfur dioxide, or SO2, in wine. One source is from yeast. Different strains of yeast can produce anywhere from 10 ppm to 80 ppm during primary alcoholic fermentation. Most commercial strains for winemaking produce only 10 – 30 ppm, so they are generally preferred. The amount of SO2 produced by a given strain of yeast is also influenced by certain chemicals such as methionine and cysteine, but since these nutrients come from the grape juice itself, we need not be concerned about them. The second source of SO2 is from additions made by the winemaker.
Second, what does SO2 do? At the levels usually desired in wine, sulfur dioxide does several things. When added early to grapes or juice, especially with white wines, it inhibits some of the enzymes that cause browning. This can be especially helpful if a pre-inoculation “cold soak” is planned for a delicate white wine such as a Muscat. Another reason for addition of SO2 before primary fermentation is to control “wild” yeasts that are present on the grapes, but are not species usually found helpful in fermentation to wine alcohol levels. Sulfur dioxide added before primary fermentation, after primary fermentation, or after malolactic fermentation can be used to control the growth of bacteria. But note, if malolactic fermentation is desired, addition of sulfur dioxide before malolactic inoculation must be made at relatively low levels since malolactic bacteria are inhibited at moderate levels of Free SO2.
Third, where does sulfur dioxide go? Not all of the SO2 that starts off in the wine stays in the wine. Some, but not much, is given off as a gas. Some gets bound to other chemicals in wine, such as acetaldehyde and anthocyanins and even residual sugars. Some chemically reacts with oxygen and other “oxidizing substances.” And some, the “Free SO2,” stays around to work. There are a couple of other points to make here. Most of the bound sulfur dioxide, which can be 50 – 90% of the amount of SO2 added, is not available to function as a preservative. It is part of the “Total SO2” in a wine, but is effectively of no use as a protectant against oxidation or against bacteria. That is why most measurements are for “Free SO2.” Also, since sulfur dioxide protects against oxidation, especially of red wines, why not just add a bunch as long as you can’t taste it? The problem here is that too much SO2 ties up the aldehydes in wine, a chemical family that slowly reacts with tannins over time to produce the desired improvements in a wine’s taste. You wouldn’t have oxidized wines, but you would have overly astringent wines instead. That’s part of the reason for the upper end on the target levels for Free SO2.
Fourth, what’s the big deal with pH? Well, let’s start with a little chemistry. The sulfur dioxide in wines exists in two different chemical forms, SO2 and HSO3-1 . These two chemical species are called “molecular SO2” and “bisulfite,” respectively. When you measure Free SO2 you measure both the molecular SO2 and the bisulfite, but only the molecular SO2 is effective as a bacterial preservative! And in wines, only 1% to 7% of the Free SO2 is present as molecular SO2. These two chemical forms are related in the following chemical equation:
SO2 + H2O = HSO3-1 + H+1
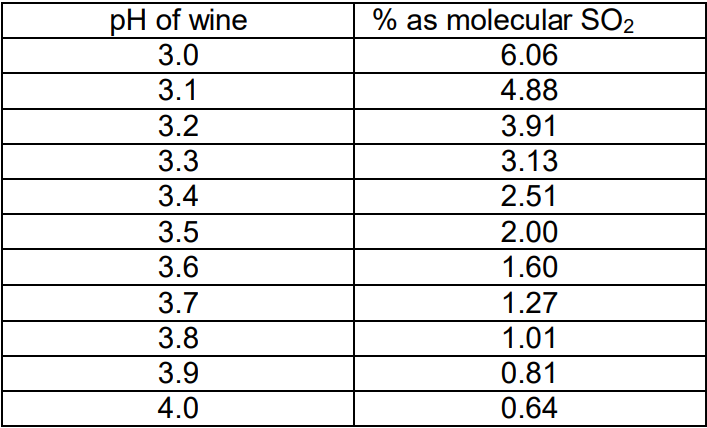
What this equation says is that if you have more acid, which is the same as having a low pH wine, then you get a higher percentage of the molecular SO2. If you have less acid, or a high pH wine, you get a much lower percentage of the molecular SO2. The following table shows the percent of the Free SO2 that is present as molecular SO2 for different pH levels in wine.
What the table says is that you need 9.45 times as much Free SO2 if your wine has a pH of 4.0 as you would if the same wine had a pH of 3.0. Wow! Do I have to understand all this chemistry to make wine? Not exactly, but it’s helpful to understand the concepts given above, then use the included chart below to actually calculate your additions.
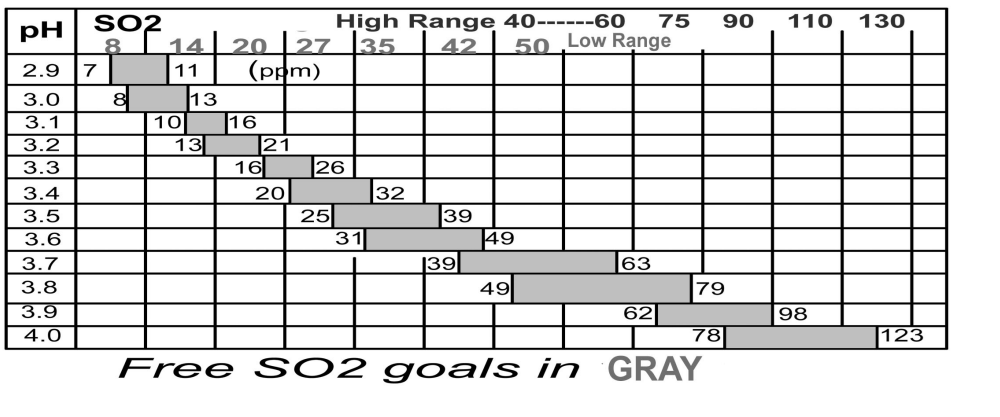
Now I know how much is needed, but how do I make my additions? First, measure the pH. If the pH is not within the range you want and you are planning a pH adjustment, make the pH adjustment before you add your SO2. Now consult the chart for the pH of your wine, then pick the target SO2 level you want. For example, if your red wine has a pH of 3.4, you’ll choose a target of 32 ppm, and use this figure in your addition calculation. However, if you added some sulfur dioxide previously you’ll need to perform a Free SO2 test to see what level is present in the wine. If the analysis says you have 10 ppm already present in the wine, then you subtract this from the target amount, and get 32 ppm – 10 ppm = 22 ppm as the amount to add.
O.K., so just how much is 22 ppm. If you will be using potassium metabisulfite for your addition, you can use the following formula:
grams of metabisulfite to add = ______ppm x 0.00657 x _______gallons of wine, juice or must
Thus, for a 22 ppm addition to 5 gallons of wine, you will add: 22 x 0.00657 x 5 = 0.72 g of metabisulfite.
Remember that SO2 tends not to mix, but stays where it is added in juice or remains in a layer near the bottom in wine. Always stir thoroughly, then stir again the next day. Also, because some of the added sulfur dioxide will become bound and unavailable, wait 2-3 days before retesting so that free SO2 and bound SO2 have a chance to equilibrate, then retest and make another addition if needed. For these reasons it is recommended to make adjustments at least a few days before bottling. (Some winemakers making smaller batches may find it convenient to use Campden tablets instead of potassium metabisulfite. The calculation to use is the same. Just remember that each tablet contains 0.44 g of potassium metabisulfite. Therefore, in the example above, you would use two Campden tablets.)
References
1. Y. Margalit, “Concepts in Wine Chemistry,” The Wine Appreciation Guild, San Francisco 1997.
2. B. W. Zoecklein, K. C. Fugelsang, B. H. Gump, and F. C. Nury, “Wine Analysis and Production,” Chapman & Hall, New York 1995.
3. R. B. Boulton, V. L. Singleton, L. F. Bisson, and R. E. Kunkee, “Principles and Practices of Winemaking,” Plenum Publishers, 1998.
4. T. Hennick-Kling and Y. H. Park, “Considerations for the Use of Yeast and Bacterial Starter Cultures: SO2 and Timing of Inoculation,” Am. J. Enol. Vitic., 45 (4) 464 1994.
5. R. Eschenbruch, Sulfite and Sulfide Formation During Winemaking – A Review,” Am. J. Enol. Vitic., 25 (3) 157 1974.